India CDSCO Certification Service
General Information:
The Indian medical device market is $11.5 billion with a projected growth rate of 22%, and imports are around 75% of the total market size.
The Central Drugs Standard Control Organization (CDSCO) regulates medical devices in India. Up until now only 37 products are regulated (notified) under CDSCO’s licensing scheme. Most of these products are implantable devices (e.g. Catheters, Stents, Valves, Implants, etc.). As of October 1st 2022, all Class A and B medical devices are slated to become regulated under the CDSCO licensing scheme. Medical devices are classified by risk in accordance with the table below:
| Class | Risk Level |
|---|---|
| A | Low Risk |
| B | Low Moderate Risk |
| C | Moderate High Risk |
| D | High Risk |
CDSCO maintains lists of medical products and their associated classifications on their website. For medical products not included on one these lists, we would recommend holding off on registering with CDSCO until that product’s classification has been clearly defined. These new CDSCO requirements will encompass many new product types, and there is certain to be confusion at the certification body, test laboratory, and enforcing agency levels. Based on our experience in dealing with Indian certifications, G&M considers these un-written but real-world issues to be part of our responsibility to help our customers navigate. It can be an ever-changing landscape, but it helps to have the support of a guide who knows where many of the rough patches lie.
As of October 1st, 2022, products that fall under a mandatory list as Class A or B must obtain a license from CDSCO to be operated in India. The typical license process is as follows
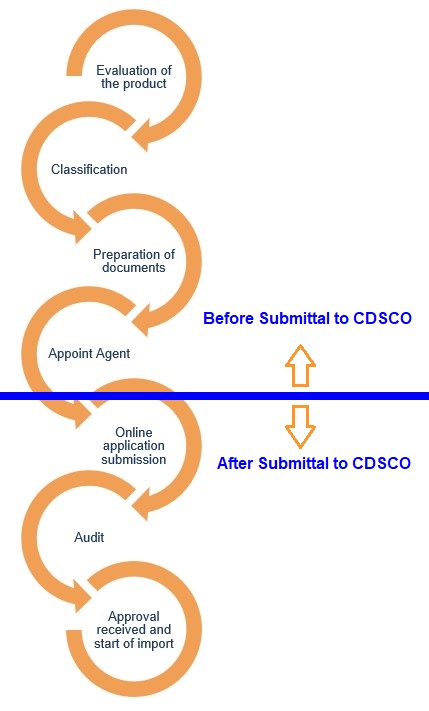
In order to comply with CDSCO requirements, medical devices shall conform to standards adopted by the central government. If such standards are not available then ISO, IEC, or any other standards may be utilized. Once certification is granted CDSCO will issue a license number. That number must be marked on the medical product to which it was assigned. From October 1st, 2023, all Class C and D medical devices (presently non-notified) are slated to become mandatory under the CDSCO licensing scheme.
G&M Compliance has operations in the U.S. and India to ensure your medical products move through certification efficiently. Although these requirements are somewhat new for the Indian market, our compliance experts have years of experience with medical device requirements for both the U.S. and European markets. Our experience with the technical requirements along with our knowledge of BIS, WPC, TEC and other Indian certification schemes, makes us the ideal partner for quick and effective CDSCO certification. For more information, additional questions, or to request a quote – Please submit an online RFQ, Contact Us, or call.


